Empirical Formula and Molecular Formula
June 2016 AS June 2017 A-level QAN code. The empirical formula for trichloroisocyanuric acid the active ingredient in many household bleaches is OCNCI.
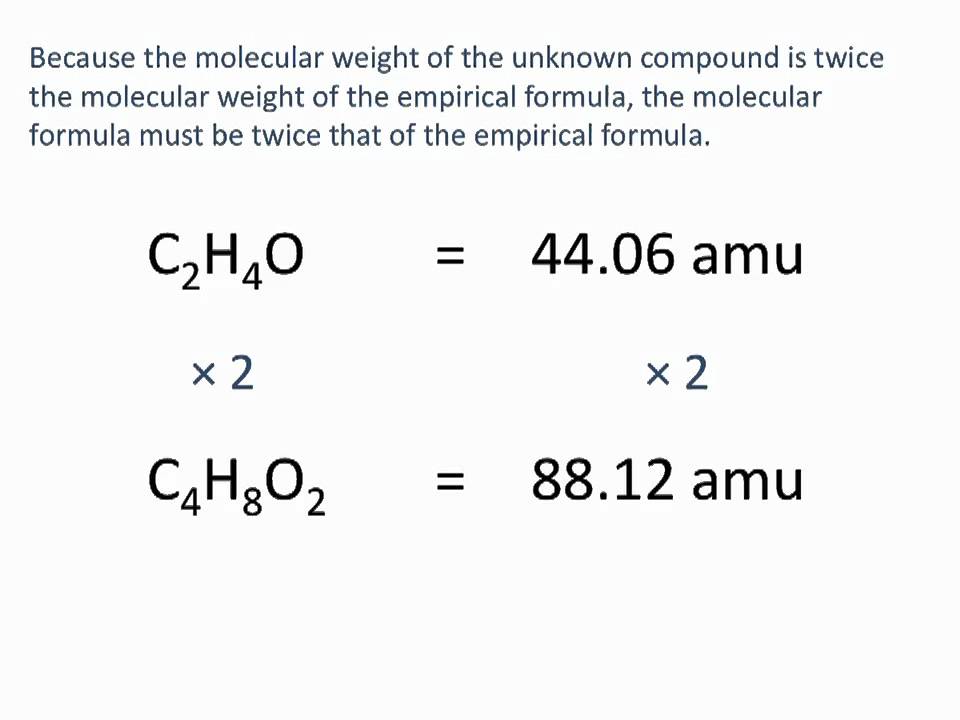
Determining Empirical And Molecular Formulas Chemistry Tutorial Youtube Chemistry Education Chemistry Molecular
The empirical formula and molecular formula are related by a whole number ratio.

. What is a molecular formula vs empirical formula. Degree of Unsaturation Formula - Meaning Solved Examples and FAQs. Multiply every atom subscripts by this ratio to compute the molecular formula.
Molecular Weight Formula - Meaning Calculation and Solved Examples. Empirical Formula - Meaning Examples Statistics and FAQs. To find the ratio between the molecular formula and the empirical formula.
The final product is a series of chemical reactions simplified below as- The rusting of the iron formula is simply 4Fe 3O 2 6H 2 O. As the empirical formula shows the simplest ratio of atoms and molecular formulas display the actual number of atoms in a molecule and structural formulas show how the atoms are bonded to each other. Structural information is how the atoms are connected and how the molecule fills the space.
The simplest formula of polyethylene is C_2H_4_n. For example our made up empirical formula would look. Those three whole numbers are the little numbers that hang at the foot of each letter that represents a separate element of the compound.
What is the empirical formula of polyethylene. Empirical formula Empirical formula of a compound is the chemical formula is the simplest ratio of Q. Empirical definition derived from or guided by experience or experiment.
A Molecular Approach 4th Edition 4th Edition Nivaldo J. The chemical formula of a compound is defined as the symbolic representation of the composition of a compound. But it is not possible always.
For the spectrum on the right a solution of 0249 mg of the unsaturated aldehyde in 95 ethanol 142 10-5 M was placed in a 1 cm cuvette for measurement. An acid is a substance that gives H ions in its solution whereas a basic substance gives OH- ions. Using the above formula ε 36600 for the 395 nm peak and 14000 for the 255 nm peak.
Lead IV Oxide Formula - Structure Production Properties and Example. The whole number ratio that we just solved actually fits in to the empirical formula. Empirical Formula Concept of Empirical Formula.
It may be the same as the compounds molecular formula sometimes. In some cases the empirical formula is the same as the molecular formula which gives the actual number of atoms in a compound eg H 2 O. Basically the mass of the empirical formula can be computed by dividing the molar mass of the compound by it.
Tro Chapter 3 Problem 49E. Otherwise the molecular formula is a multiple of the empirical formula eg CH 2 O is. Express your answer as a chemical formula.
An example of the difference is the empirical formula for glucose which is CH 2 O ratio 121 while its molecular formula is C 6 H 12 O 6 number of atoms 6126. What is the molecular formula of trichloroisocyanuric acid. Caffeine Chemical Formula - Meaning Properties and FAQs.
Ethyl Alcohol Structural Formula. The formula mass formula weight of glucose is 30 either no units or else grams per mole while the molecular mass molecular weight is 180156 gmol. A molecular formula provides more information about a molecule than its empirical formula but is more difficult to establish.
The molecular formula describes the composition of ethanol molecules two carbon atoms six hydrogen atoms and one atom of oxygen occur per molecule but gives us no structural information. Structural representations give even more information than molecular formulas. For water both formulae are H 2 O.
Determine the molecular formula of a compound with an empirical formula of NH2 and a formula mass of 3206 amu. In addition to showing how many of each atom is present in a molecule structural representations give you information about the bonding and structure of the. The formula mass and molecular mass of water H 2 O are one and the same while the formula and molecular mass of glucose are different from each other.
Textbook solution for Chemistry. The chemical formula for rust is Fe 2 O 3 and is commonly known as ferric oxide or iron oxide. A compound contains 8879 oxygen O and 1119.
The molar mass of this compound is 23241 gmol. We have step-by-step solutions for your textbooks written by Bartleby experts. The empirical formula of any compound is the simplest whole-number ratio of each individual type of atom in that compound.
Understand what those whole numbers mean for the empirical formula. We may calculate the empirical formula from information about the mass of.

Empirical Vs Molecular Formula Molecular Chemistry Study Guide Chemistry Education

Determining Empirical And Molecular Formulas Chemistry Tutorial Youtube Chemistry Education Chemistry Molecular

Unit 6 Molecular And Empirical Formula Sheet Chemistry Classroom Chemistry Lessons Chemistry 101

Empirical And Molecular Formula Notes Chemistry Worksheets Teaching Chemistry Chemistry Notes
0 Response to "Empirical Formula and Molecular Formula"
Post a Comment